On November 30, 2023, Nature Biomedical Engineering (Nature BME) published two back-to-back papers [1,2] online by the research team led by Academician Lihong V. Wang from the California Institute of Technology (Caltech), reporting novel photoacoustic imaging-based methods for hemodynamic measurements. On December 27, the same journal featured a commentary article [3] by the team of Researcher Chengbo Liu from the Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences, which analyzed the working principles, scientific value, and implications of the two studies, while also outlining future directions for the technology.
Figure 1. News and Views commentary article
Hemodynamic monitoring is key to understanding human physiological and pathological states, and its visualization provides critical information for assessing life functions and diagnosing vascular diseases. Abnormal blood supply causes severe damage to tissues and organs—for example, abnormal cerebral blood flow can trigger strokes, myocardial ischemia leads to heart attacks, and peripheral vascular obstruction caused by diabetes ultimately results in limb ulceration. Optical imaging techniques, including laser Doppler and laser speckle technologies, can achieve high-resolution blood flow imaging, but their clinical applications are limited by penetration depths (approximately 1 mm). Photoacoustic imaging, particularly photoacoustic computed tomography (PACT), effectively addresses this issue. By converting light energy into acoustic energy, PACT extends imaging depths to the centimeter scale, enabling deep-layer blood flow imaging. However, PACT has faced prolonged challenges in advancing deep-layer hemodynamic imaging, primarily due to the following limitations. First, PACT images lack speckle effects, making it difficult to measure flow signals within vascular lumens. This arises from the random cancellation of photoacoustic signals generated by numerous red blood cells in the vascular lumen; thus, conventional PACT can only visualize vascular boundaries. Second, in traditional PACT imaging, a trade-off exists between imaging speed and system complexity: existing technologies either sacrifice imaging speed by scanning with a single detector or significantly increase system complexity and cost by using multiple detectors for parallel detection.In two back-to-back papers published online in Nature BME on November 30, the team led by Academician Lihong V. Wang reported two novel technologies: photoacoustic vector tomography (PAVT) [1] and photoacoustic computed tomography via ergodic relay (PACTER) [2], which effectively overcome PACT's limitations in deep-layer blood flow imaging and represent a major breakthrough for photoacoustic imaging in hemodynamic monitoring.
In the PAVT technology [1], researchers achieved deep-layer blood flow imaging by leveraging spatial heterogeneity within blood. They astutely observed that the absence of speckle phenomena does not apply to all blood vessels. In scenarios involving non-uniform blood flow and blood pressure fluctuations—such as pressure variations in venous flow caused by smooth muscle contractions and unidirectional valve operations—detectable speckle signals are induced within the blood (Figure 2a). Based on this discovery, the team developed PAVT, successfully visualizing intravascular blood flow dynamics. To estimate flow velocity and direction, the researchers further analyzed correlations between consecutive image frames (Figure 2b), presenting hemodynamic information as 2D blood flow velocity vector maps (Figure 2c).
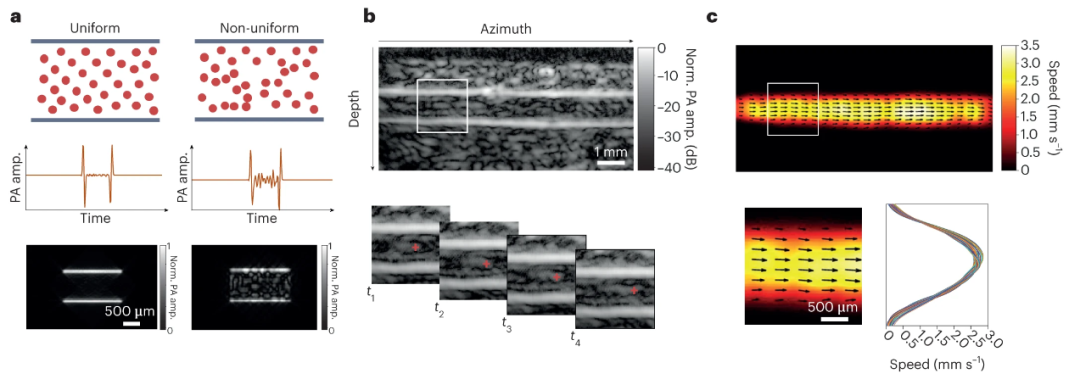
Figure 2. PAVT technology. a, Dependence of intravascular signals on blood heterogeneity. In homogeneous blood, the amplitude of intravascular signals is nearly invisible; in heterogeneous blood, intravascular signals exhibit sufficiently high amplitude visibility. b, Real-time reconstruction of vascular structures. The magnified view of the white rectangular region analyzes correlation between consecutive image frames. c, First row: Flow vector field overlaid on a 2D blood flow velocity map. Second row: Magnified view of the white rectangular region and its corresponding velocity profiles.
While PAVT technology achieves vector blood flow imaging for the first time, it is limited to two-dimensional images. To overcome this challenge, the researchers further developed a single-point detection technology capable of ultrafast 3D blood flow imaging, termed PACTER [2] (Figure 3). This technology first employs point-scanning imaging to calibrate the system, utilizing an ergodic relay (ER) to spatiotemporally encode photoacoustic signal waveforms (Figure 3a–c): Each optical focus generates a photoacoustic signal modulated by its unique propagation path in the ER cavity, endowing it with distinct features. In this setup, when a diverging light beam excites the entire imaging target, the resulting photoacoustic signal becomes a linear combination of signals from all focal points in the point-scanning mode. A linear scaling relationship is then applied to extract photoacoustic signals at each focal point.Building on this, the researchers introduced the concept of a virtual detector (VD) (Figure 3d). By calculating the time delay of photoacoustic signals from different voxels in the imaging target to the VD (Figure 3e), they reconstructed the 3D spatial positions of signals, enabling 3D blood flow imaging. This method not only acquires 3D images of the target with a single laser pulse but also circumvents the limitations of conventional PACT, such as high system complexity, cost, and size. Using PACTER, the team achieved a 3D imaging rate of 1 kHz, making rapid hemodynamic imaging feasible.
Although ultrasound Doppler imaging can be used to obtain deep-layer blood flow information, PAVT retains advantages in hemodynamic imaging by comparison. PAVT not only provides optical absorption contrast but is also unaffected by detection angle limitations. This enables PAVT to be applied to broader scenarios, such as imaging microvessels with small diameters and low flow velocities. Compared to PAVT, PACTER elevates hemodynamic imaging to a new level. By leveraging spatiotemporal encoding and the virtual detector (VD) concept, this technology achieves single-point-detection-based rapid 3D imaging, offering significant reference and scalability for applications constrained by detection volume limitations—for example, in portable home care monitoring and wearable medical devices.
In summary, the two novel technologies reported by the team led by Academician Lihong V. Wang provide a new paradigm for non-invasive, label-free deep hemodynamic imaging in humans. These technologies not only facilitate precise clinical diagnosis of vascular diseases but also hold future potential for brain function studies when integrated with blood oxygen metabolic imaging. Nevertheless, further optimizations are required. For example, PAVT currently enables imaging of venous blood flow but struggles with arterial blood flow lacking significant spatial heterogeneity. While PACTER has achieved 3D hemodynamic imaging in both small animals and humans, its imaging quality still needs improvement. With ongoing research—such as addressing acoustic impedance mismatch between the imaging target and the ergodic relay (ER), as well as expanding the physical coverage of the ER—both technologies are poised for promising translational applications.
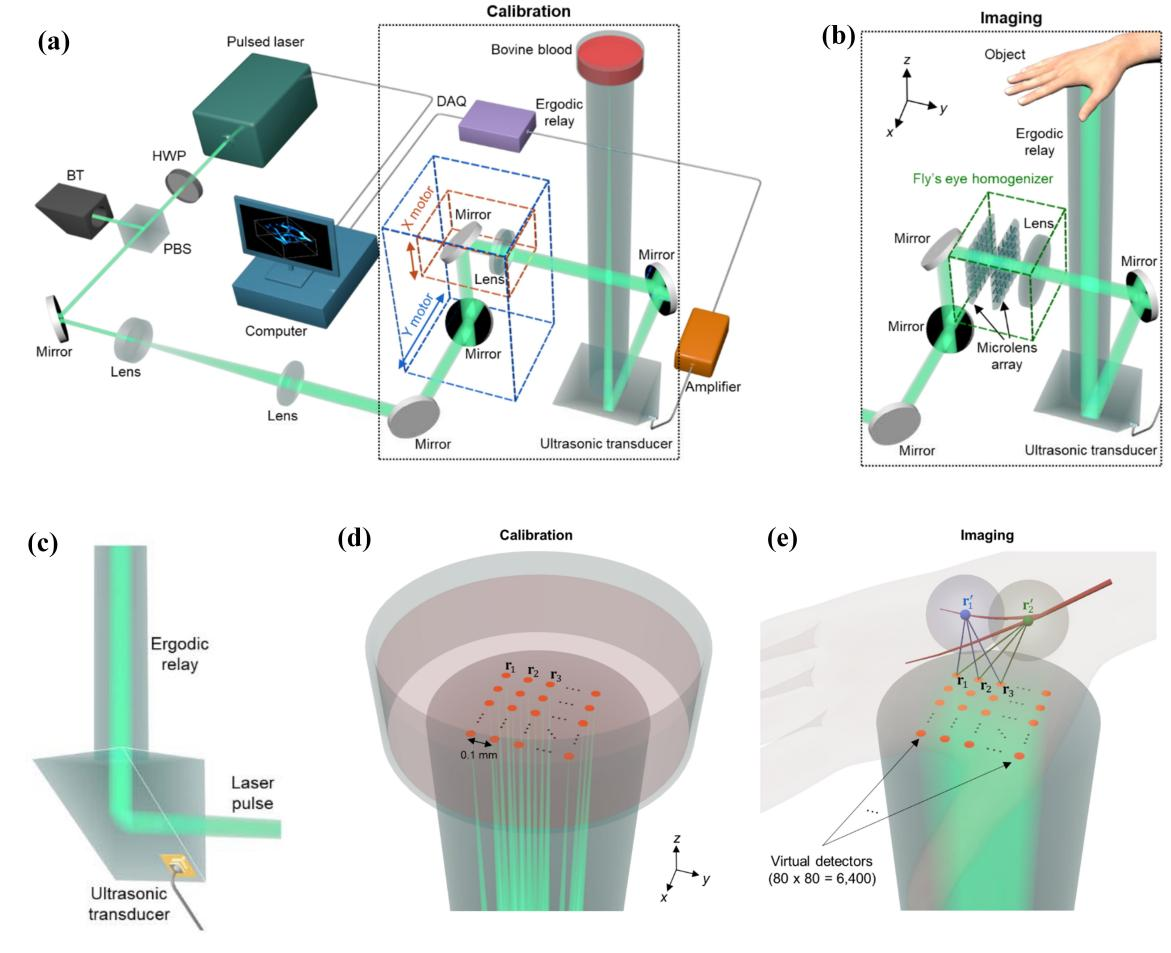
Figure 3. PACTER technology. a, b, Schematic diagrams of single-point-scanning calibration (a) and divergent light beam imaging (b), with dashed boxes highlighting their differences. c, Single-element ultrasound transducer detection based on the ergodic relay (ER). d, Focused beam (green) and pixels (orange) during single-point-scanning calibration, with the latter representing the virtual detector (VD). e, PACTER imaging of human hand vasculature using a divergent light beam (green) after calibration. r1’ and r2’ denote photoacoustic signal sources generated within blood vessels. r1, r2, and r3 represent signals captured by the VD with distinct time delays from the sources (r1’ and r2’).
References:
1. Yang Zhang, Joshua Olick-Gibson, Anjul Khadria, Lihong V. Wang. Nature biomedical engineering. https://doi.org/10.1038/s41551-023-01148-5.
2. Yide Zhang, Peng Hu, Lei Li, Rui Cao, Anjul Khadria, Konstantin Maslov, Xin Tong, Yushun Zeng, Laiming Jiang, Qifa Zhou, and Lihong V. Wang. Nature biomedical engineering. https://doi.org/10.1038/s41551-023-01149-4.
3. Rongkang Gao, Zhiqiang Xu, Chengbo Liu. Nature biomedical engineering. https://doi.org/10.1038/s41551-023-01162-7.