Recently, the research team of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences and the University of Texas at Austin in the United States jointly published research results in Proceedings of the National Academy of Sciences of the United States of America(PNAS), proposing a new strategy for the diagnosis and treatment of rheumatoid arthritis based on a biomimetic delivery system.
Screenshot of the article's online release
In this study, small interfering RNA (siRNAT/I) and Prussian blue (PB) were used in combination to silence the expression of pro-inflammatory cytokines TNF-α/IL-6, eliminate the overexpressed reactive oxygen species (ROS) in the rheumatoid arthritis (RA) microenvironment, and simultaneously alleviate the hypoxic state. In order to improve the in vivo stability, biocompatibility, and inflammation-targeting ability of siRNAsT/I and PB, the researchers extracted endogenous macrophages and then stimulated them to produce homologous membrane vesicles as biomimetic carriers, and synthesized a comprehensive platform with integrated diagnosis and treatment functions—M@P-siRNAsT/I (Figure 1).
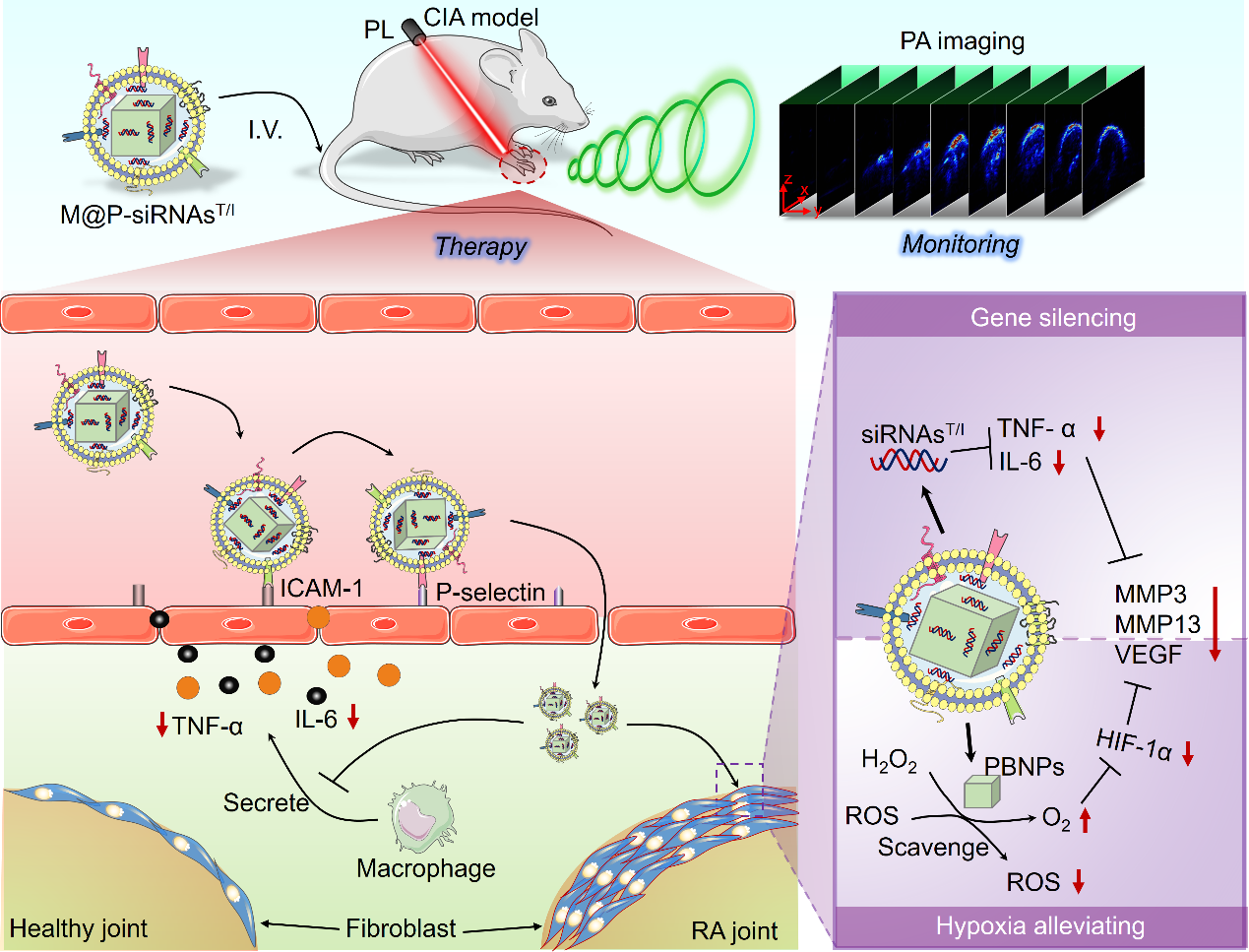
Figure 1. Schematic diagram of the targeted diagnosis and treatment research for rheumatoid arthritis
In terms of the treatment strategy for rheumatoid arthritis (RA) in this study, it breaks through the traditional thinking. Aiming at the complex and diverse inflammatory microenvironment, it innovatively inhibits the expression of multiple genes related to pro-inflammatory factors. At the same time, it regulates the RA inflammatory microenvironment, eliminates reactive oxygen species (ROS), relieves hypoxia, etc., achieving a "two-pronged" therapeutic effect. In terms of the targeted recognition of the RA microenvironment, the natural inflammation-tropic characteristics of the endogenous biomimetic delivery system are utilized to solve the dilemma of easy off-targeting that exists in the traditional specific recognition strategy based on donors and receptors. In terms of imaging guidance and treatment evaluation, photoacoustic imaging technology is adopted to solve the problem of shallow penetration and lack of depth information in fluorescence imaging during joint imaging. It can obtain real-time three-dimensional molecular information of joint lesions, providing image information for the precise in-vivo treatment of RA. At the same time, it can also monitor the changes in functional information such as blood oxygen during treatment, providing an effective imaging method for the prognosis evaluation of RA. The first author of this paper is Dr. Chen Jianhai, a postdoctoral fellow jointly trained by Shenzhen Institutes of Advanced Technology and the Second Affiliated Hospital of The Chinese University of Hong Kong (Shenzhen Longgang People's Hospital). The corresponding authors are Associate Researcher Chen Jingqin and Researcher Liu Chengbo from Shenzhen Institutes of Advanced Technology, and Professor Jonathan L. Sessler from The University of Texas at Austin, USA. Special thanks are given to Researcher Zhang Peng and Researcher Song Liang from Shenzhen Institutes of Advanced Technology, Dr. Adam from the University of Oxford, Professor Wang Benguo from Longgang People's Hospital, Professor Zhang Hai from Shenzhen People's Hospital, and Professor Fang Chihua from Southern Medical University for their guidance and strong support. This work was supported by the National Natural Science Foundation of China, the Key R&D Program of the Ministry of Science and Technology, the Key Laboratory of Health Informatics of the Chinese Academy of Sciences, the Key Laboratory of Molecular Imaging of Guangdong Province, and the Robert A. Welch Foundation of the United States.