Neurovascular coupling (NVC) is a functional mechanism through which neural activity dynamically regulates cerebral blood flow. When neurons become active, nearby blood vessels dilate to increase blood supply, meeting the energy demands of neural processes. This mechanism is fundamental to maintaining normal brain function and is crucial for non-invasive brain-computer interfaces to acquire brain information.However, traditional techniques have struggled to achieve real-time, high-precision observation of neuronal and vascular activities across the whole brain due to limited detection areas (typically <1 mm²) or insufficient spatiotemporal resolution, constraining further exploration of NVC mechanisms.
On July 23, a research team led by Academician Zheng Hairong, Researcher Liu Chengbo, and Researcher Zheng Wei from the National Key Laboratory of Medical Imaging Science and Technology Systems at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, announced a major technological breakthrough. Building on their first-generation head-mounted microscopic imaging system (published in Science Advances, 11, eadu1153, 2025), they successfully developed a high-speed, wide-field hybrid photoacoustic and fluorescence microscopic imaging system (LiTA-HM). This system enables simultaneous, dynamic, and high-resolution imaging of neuronal activity and multiple microvascular parameters across the entire cortex of live mice, offering a new paradigm for data acquisition in non-invasive brain-computer interfaces.
The findings were published in Science Advancesunder the title "Photoacoustic and fluorescence hybrid microscope for cortex-wide imaging of neurovascular dynamics with subcellular resolution." The journal concurrently published a Focus article by Deputy Editor Prof. Junjie Yao of Duke University, which highlighted the study and remarked: “LiTA-HHM integrates neural activity and vascular physiology within a single imaging framework, combining spatial breadth and temporal precision, making it possible to decipher the most fundamental neurovascular collaborative mechanisms in the brain. This breakthrough brings the academic community one step closer to a long-standing goal: observing the rhythm of brain function and thought in real time, with single-neuron and single-vessel resolution.”
The research was supported by the Chinese Academy of Sciences Pioneer Program B Project (XDB0930000) and the National Key Laboratory of Medical Imaging Science and Technology Systems, providing key technologies for subsequent work under the Pioneer B Program. The co-first authors of the paper are Liu Liangjian (Ph.D. student at Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences), Associate Researcher Xu Zhiqiang, Lai Zhenjie (Master's student at Shenzhen University), and Associate Professor Xu Bin. Academician Zheng Hairong, Researcher Liu Chengbo, and Researcher Zheng Wei served as corresponding authors. Collaborative teams included researchers from the Shenzhen Institute of Advanced Technology, Hong Kong Polytechnic University, Naval Medical University, and Qufu Normal University.
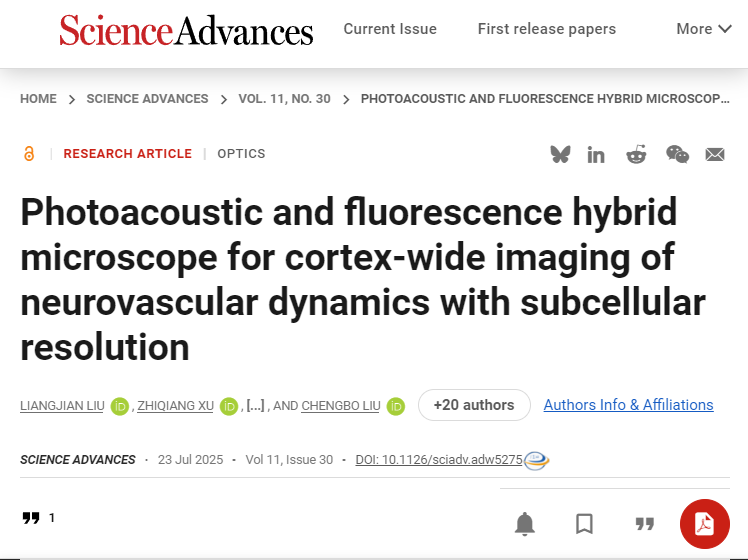
The research team developed polygonal mirror and array transducer technology, successfully creating a high-speed, wide-field photoacoustic/fluorescence multimodal microscopic imaging system. This instrument innovatively integrates optical-resolution photoacoustic microscopy (OR-PAM) with confocal fluorescence microscopy (CFM), enabling simultaneous acquisition and precise registration of dual-modal images at the hardware level. Utilizing advanced scanning and detection technologies, the system overcomes the traditional trade-offs among imaging field of view, resolution, and speed. It achieves a spatial resolution of 6 μm, a field of view of 6 mm × 5 mm, and an imaging speed of 1.25 frames per second, allowing real-time synchronous capture of neuronal and vascular activities across the entire cortex of mice. This breakthrough provides new opportunities for studying neurovascular coupling mechanisms and offers more comprehensive data for brain-computer interface research. A schematic diagram of the imaging system and representative results are shown below.
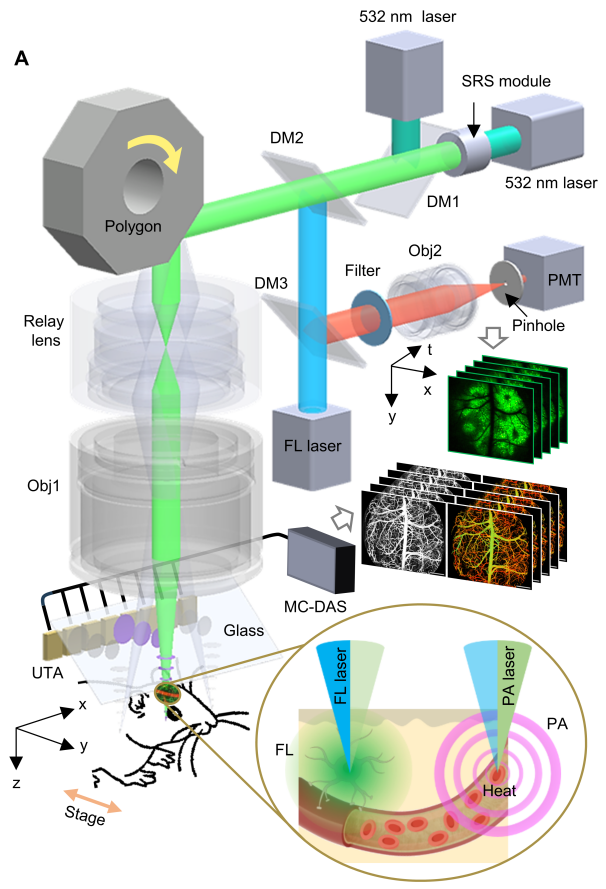
Schematic and Imaging Results of LiTA-HM
1. High-Speed, Large-Field-of-View Photoacoustic/Fluorescence Multimodal Imaging Technology
High-Speed, Large-Field-of-View, and High-Sensitivity Detection Technology: This study innovatively employs an 8-channel array transducer to detect photoacoustic signals, maintaining exceptionally high sensitivity even within a large 6 mm imaging field of view. The detection technology is seamlessly integrated with high-speed polygonal mirror scanning, allowing the mirror to operate in air without interference from ultrasound coupling media. This design ensures both high imaging speed and system stability.
Advanced Image Reconstruction Algorithm: The team developed a novel photoacoustic image reconstruction algorithm tailored for array transducer detection. By incorporating weighted averaging and adaptive stripe filtering with correction techniques, the algorithm significantly enhances image signal-to-noise ratio while effectively eliminating brightness and dark stripe artifacts introduced by array detection.
Optical Imaging Optimization: Through optical simulations, the team optimized the polygonal mirror and its scanning pathway system. Within the 6 mm imaging field, the system achieves a consistent resolution of approximately 6 μm and a focal depth of 292 μm, providing critical technical support for large-field-of-view photoacoustic/fluorescence multimodal imaging.
Integrated System Innovation: Leveraging advancements in optics, key components, hardware/software systems, and imaging algorithms, the research team developed the LiTA-HM high-speed large-field-of-view photoacoustic/fluorescence multimodal microscopy system. This system enables simultaneous whole-brain acquisition of neural activity and hemodynamic parameters. It demonstrates exceptional imaging performance, capturing capillary-level vascular networks and individual neuronal somata across the entire cortical field of view, as illustrated in the figure below.
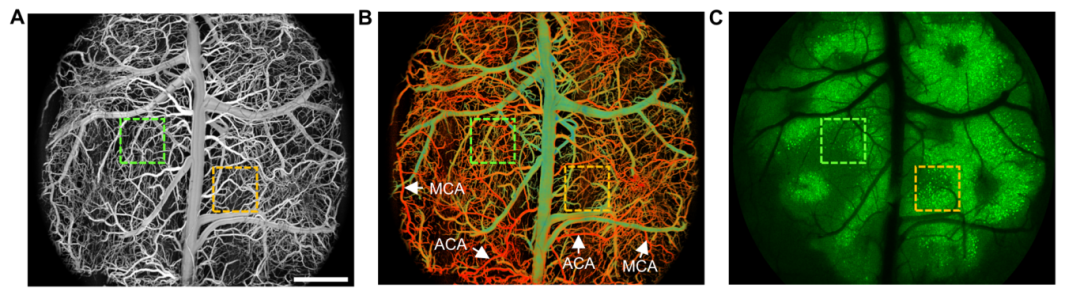
Imaging Results: (A) Whole cerebrovascular structure of the mouse brain; (B) Vascular oxygen saturation map; (C) Neuronal cell bodies map. Scale bar = 1 mm.
2. Multi-scenario Applications Reveal Spatiotemporal Characteristics of Neurovascular Coupling
Based on LiTA-HM, the research team successfully conducted imaging experiments on brain diseases and neural functions in awake mice, demonstrating the technology’s potential for both research and application in neuroscience.
Study on Neurovascular Coupling During Epileptic Seizures: The team established a pentylenetetrazole (PTZ)-induced mouse model of epilepsy to investigate neurovascular coupling during seizures. This study successfully captured the propagation process of cortical spreading depression (SD) across neuronal and vascular networks under a whole-brain field of view. As shown in the figure below, when SD waves propagated separately in the left and right hemispheres, neuronal calcium dynamics and cerebrovascular blood perfusion fluctuations showed significant spatial and temporal correlations. The results suggest that monitoring cerebral hemodynamic changes could potentially identify the seizure focus and predict the timing of episodes, providing a novel approach for locating epileptic foci and predicting seizure onset in clinical diagnosis and treatment.
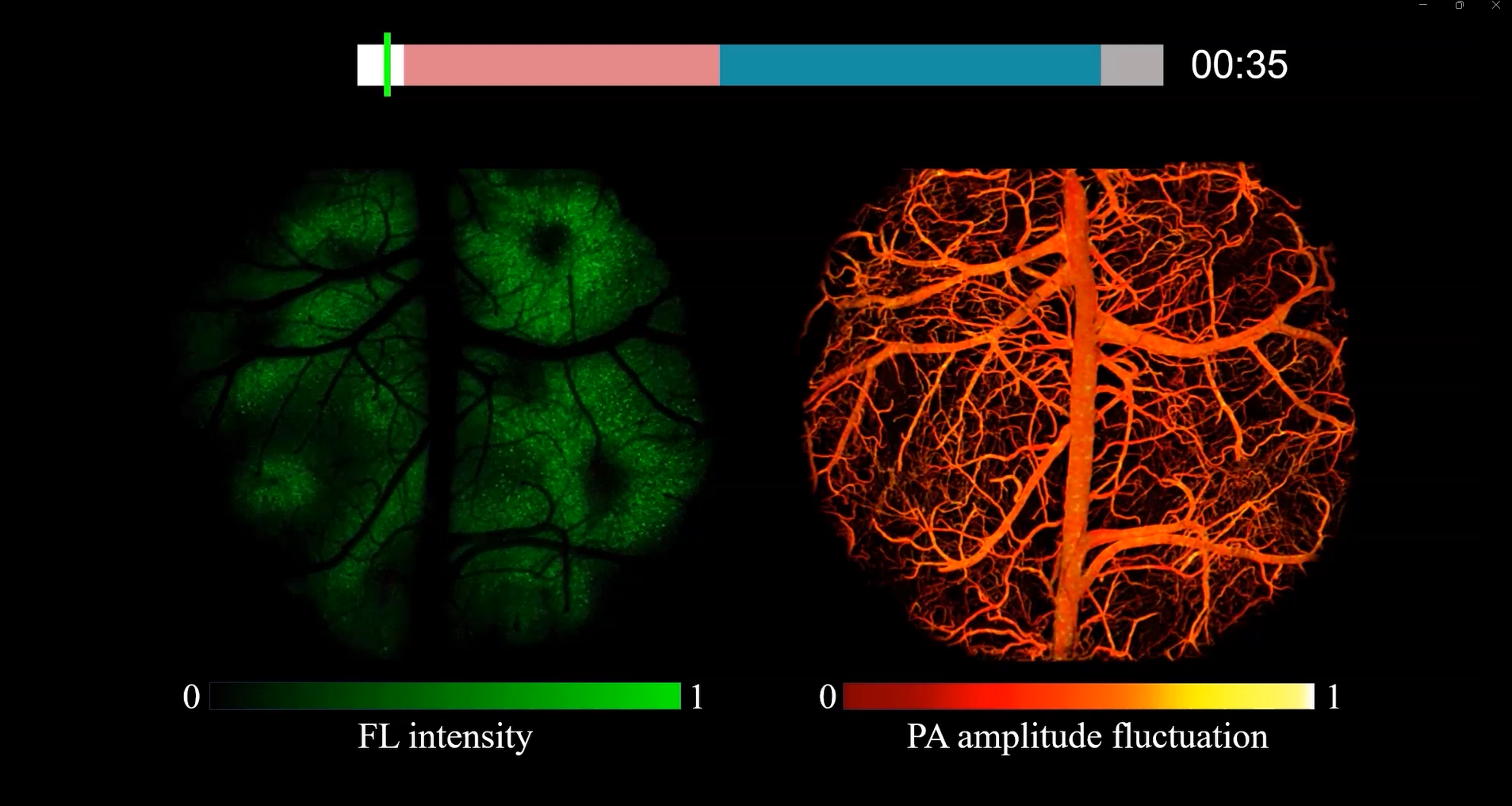
Research on Neurovascular Coupling During Epileptic Seizures
Heterogeneous Responses of Neurovascular Coupling to Hypoxia and Anesthesia: Our team investigated neurovascular coupling under hypoxia and anesthesia. The results revealed that both conditions significantly suppressed neuronal activity and induced marked vascular dilation in the cerebral cortex, but their response patterns and mechanisms differed substantially. Under hypoxia, changes in neuronal fluorescence signals lagged behind alterations in intravascular oxygen concentration, suggesting that shifts in neuronal activity were an indirect consequence of insufficient energy supply from vessels. In contrast, during anesthesia, neuronal fluorescence signals and intravascular oxygen changes were largely synchronous, likely due to the simultaneous effects of anesthetic agents on both neurons and vascular systems.
3. Future Prospects
LiTA-HM enables synchronous high-precision observation of neuronal activity and vascular networks across the entire cerebral cortex, offering new paradigms and insights for studying neurovascular coupling mechanisms and next-generation brain-computer interface (BCI) technologies.
Future research will focus on two main aspects: imaging technology and BCI applications. In terms of imaging, efforts will continue to optimize system performance, expand the field of view, and increase imaging speed, ultimately enabling high-speed imaging of the entire brain in primates. For BCI applications, this technology holds potential for non-invasive reading of brain functional information, allowing precise interpretation of brain activity based on neurovascular coupling mechanisms and providing critical scientific support for BCI research.