On November 18, a collaborative team led by Jingqin Chen and Chengbo Liu from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, together with Chihua Fang from Southern Medical University, published a research article in Nature Communications entitled “Co-delivery of sorafenib and an FSP1 inhibitor triggers dual ferroptosis in tumor cells and immunosuppressive macrophages for enhanced immunotherapy in mouse models of hepatocellular carcinoma.”
The study identifies FSP1 as a critical driver of sorafenib resistance and the formation of an immunosuppressive microenvironment in hepatocellular carcinoma. The team developed a biomimetic targeted delivery system capable of precisely directing therapeutics to both tumor cells and immunosuppressive macrophages. By inducing dual ferroptosis, the system simultaneously attacks these two key cell populations, offering a promising strategy to enhance combination immunotherapy, reverse tumor immune suppression, and address metastasis and recurrence in advanced liver cancer.
Figure 1. Online screenshot of the published article
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality worldwide. Its profoundly immunosuppressive tumor microenvironment is a major barrier to effective therapy and a key driver of recurrence. Among infiltrating immune cells, tumor-associated macrophages are highly enriched and are often polarized into an M2 phenotype, where they secrete immunosuppressive mediators, promote tumor progression and metastasis, and directly impair cytotoxic T-cell function.
Sorafenib, a standard treatment for advanced HCC, exerts its antitumor activity largely through inducing ferroptosis. However, this also triggers compensatory upregulation of alternative defense pathways—most notably FSP1—leading to robust therapeutic resistance. Complicating matters further, M2 macrophages adapt to therapy by relying on FSP1 to evade ferroptosis, allowing them to persist and maintain an immunosuppressive microenvironment even after treatment. Overcoming ferroptosis resistance in both tumor cells and M2 macrophages, while achieving precise drug delivery to both populations, may therefore be key to improving treatment responses and reversing immune suppression in HCC.
By integrating multiple single-cell RNA sequencing datasets, the study identified FSP1 as a ferroptosis-resistance driver that is specifically upregulated in sorafenib-treated HCC tissues and closely associated with M2 macrophage infiltration and poor prognosis.
Building on this insight, the team developed a biomimetic dual-targeted ferroptosis nanoplatform (Sv@PM-M2p) with three key features:
1). Homotypic targeting enabled by a hepatocellular carcinoma cell–membrane coating, facilitating selective enrichment in tumor tissue.
2). M2 macrophage targeting via M2pep peptides on the membrane surface.
3). GSH-responsive co-delivery of sorafenib and an FSP1 inhibitor (viFSP1) using a DS-PLGA polymer core, enabling controlled drug release in the tumor’s high-GSH environment.
In vitro and in vivo studies demonstrated that Sv@PM-M2p efficiently accumulates in tumors and is simultaneously internalized by cancer cells and M2 macrophages. Upon release, sorafenib and viFSP1 block the GPX4- and FSP1-mediated ferroptosis defense pathways, respectively, inducing pronounced lipid peroxidation and achieving dual ferroptosis in both cell types.
Beyond directly killing tumor cells, this approach eliminates immunosuppressive M2 macrophages, promotes their repolarization toward an M1 phenotype, activates dendritic cells and cytotoxic T cells through DAMP release, and converts an immunologically “cold” tumor into a “hot” one—thereby reshaping the entire tumor immune microenvironment.
The study further shows that treatment-induced IFN-γ upregulates PD-L1, suggesting an adaptive resistance mechanism. Accordingly, combining the nanoplatform with anti-PD-L1 therapy significantly enhances systemic antitumor immunity and suppresses primary tumor growth, distant metastasis, and recurrence across multiple mouse models.
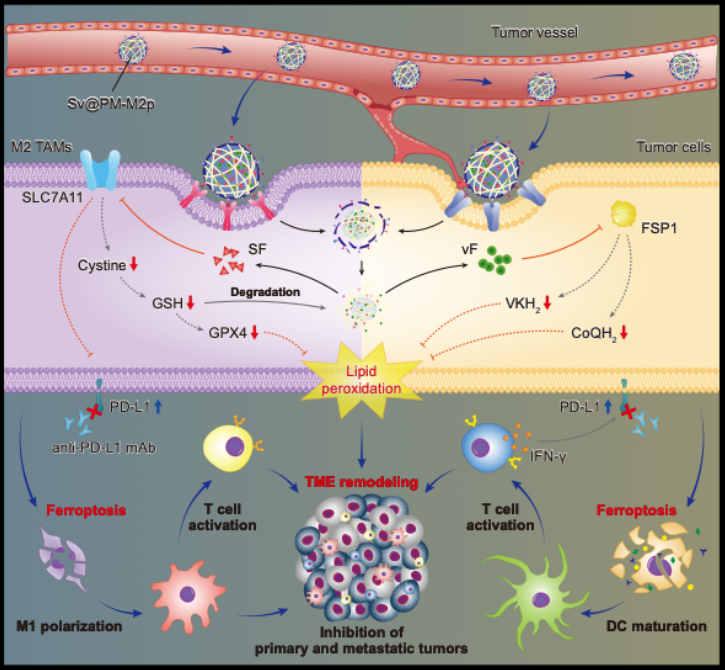
Figure 2. Schematic illustration of the study
Prof. Chihua Fang of Southern Medical University, Dr. Jingqin Chen, and Dr. Chengbo Liu of the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, served as the corresponding authors of the paper. Chuanyu Tang, a Ph.D. student at Southern Medical University and visiting student at SIAT, is the first author.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the National Key Laboratory of Medical Imaging Science and Technology, the CAS Youth Innovation Promotion Association, the Guangdong Basic and Applied Basic Research Foundation, and the Shenzhen Science and Technology Program.