Neurovascular Coupling (NVC) is a functional mechanism by which neural activity in the brain dynamically regulates local blood flow and oxygen metabolism, serving as a core target for imaging neural activity in brain-computer interface (BCI) technologies. When neurons are active, their metabolic demands increase, prompting nearby blood vessels to rapidly respond through dilation and oxygen regulation to ensure energy supply for neural activities. This mechanism is fundamental to maintaining normal brain function and critical for non-invasive BCIs to acquire brain information. However, traditional techniques struggle to achieve synchronized in vivo monitoring of brain neurons and cerebral blood flow with high spatiotemporal resolution, making it difficult to precisely correlate neuronal activity with dynamic changes in nearby blood flow and oxygen levels. Most studies are also limited to head-fixed imaging, failing to reflect NVC functions during natural behaviors.
Recently, a research team led by Academician Zheng Hairong, Researcher Liu Chengbo, and Researcher Zheng Wei from the National Key Laboratory of Medical Imaging at Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, developed a 1.7-gram head-mounted imaging microscope. This innovation achieves synchronized high spatiotemporal resolution imaging of neuronal activity and oxygen metabolism in freely moving mice, providing new insights for exploring neurovascular coupling mechanisms and developing BCI technologies. The study, titled "Simultaneous head-mounted imaging of neural and hemodynamic activities at high spatiotemporal resolution in freely behaving mice," was published in Science Advances. The research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0930000) and the National Key Laboratory of Medical Imaging Sciences and Technology Systems, providing critical technologies for follow-up work under the program. The first authors include Assistant Researcher Chen Ningbo, Associate Researcher Xu Zhiqiang, Postdoctoral Fellow Song Zheng, and Associate Researcher Liao Jiuling. Collaborating teams included those led by Researcher Zhu Yingjie at Shenzhen Institutes of Advanced Technology, Professor Puxiang Lai at The Hong Kong Polytechnic University, and Professor Kenneth K. Y. Wong at The University of Hong Kong.

Screenshot of the article's online publication
The research team achieved efficient integration and synchronization of a confocal fluorescence microscope (CFM) and a photoacoustic microscope (PAM) through miniaturized design, constructing a dual-modal imaging probe weighing just 1.7 grams. This enables synchronized neurovascular imaging with high spatiotemporal resolution in freely moving mice. The imaging resolution reaches 1.5 microns, the frame rate is 0.78 Hz, and the field of view is 400 microns × 400 microns. Through innovations in system hardware and algorithms, the team realized cerebral oxygen metabolism imaging and simultaneously recorded neuronal calcium signal activity.
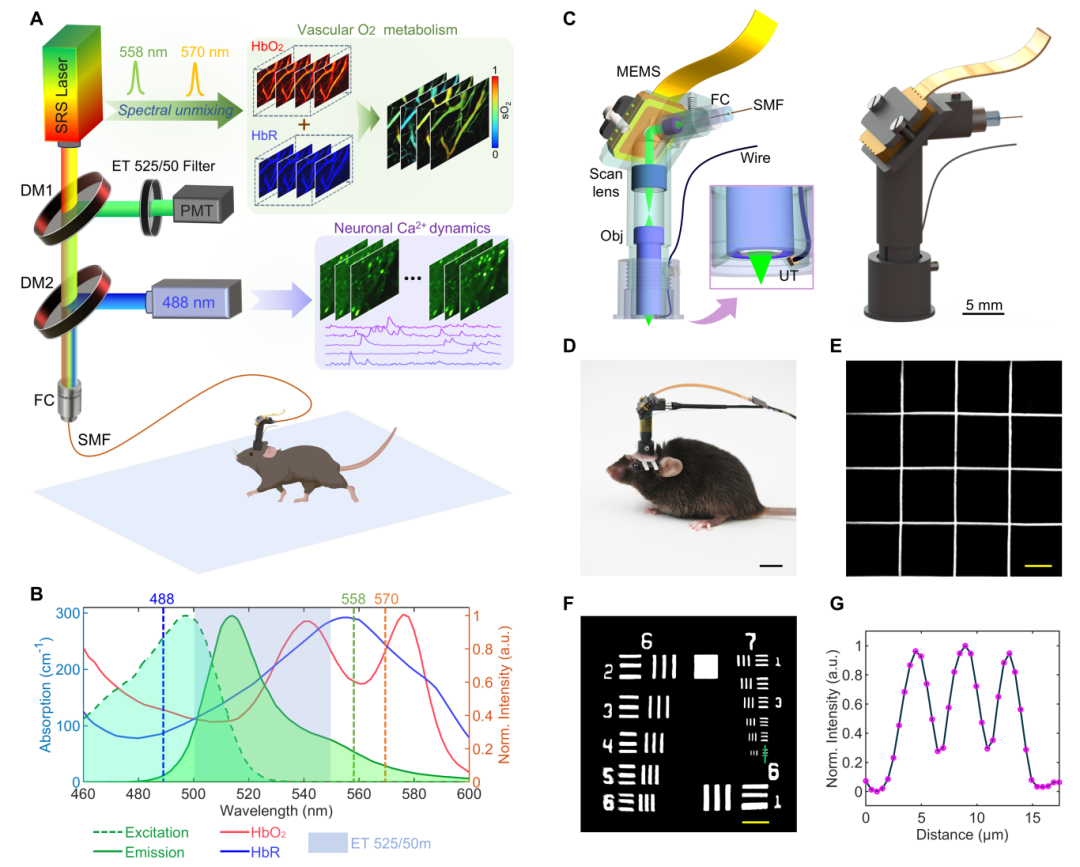
(A) Head-mounted photoacoustic/fluorescence dual-modal imaging platform; (B) Dual-modal excitation and emission spectra; (C) Miniaturized imaging probe design; (D) Head-mounted free movement of mice; (E) Imaging field-of-view calibration, scale bar: 50 μm; (F–G) Resolution calibration, scale bar: 40 μm.
1. Miniaturized Dual-Modal Imaging Technology
A core technology of this study is the lightweight integration of dual-modal imaging. Traditional optical microscopes, constrained by the size of optical components and signal acquisition complexity, struggle to balance high resolution with animal behavioral freedom, making synchronized neurovascular imaging unfeasible. The research team achieved technological breakthroughs through innovative design. 1) Multimodal Fusion
CFM tracks neuronal calcium signal activity, while PAM enables label-free detection of multi-parameter changes, including blood oxygen saturation (sO₂), total hemoglobin (HbT), oxygenated/deoxygenated hemoglobin (HbO/HbR) concentrations, and vascular diameter. 2) Lightweight Design
A high-sensitivity miniature piezoelectric ultrasonic transducer (0.4 mm×0.5 mm) replaces traditional large acoustic sensors; two-axis micro MEMS galvanometric mirrors enable imaging scanning; customized miniature objective lenses and other optical components break through the physical constraints of CFM/PAM dual-modal fusion imaging. A single optical fiber transmits three laser wavelengths (488/558/570 nm) simultaneously and collects fluorescent signals, ensuring lightweight system integration.
3) Anti-Interference Optimization
Raman fiber technology separates PAM excitation spectra from CFM emission spectra, avoiding cross-interference during simultaneous dual-modal imaging. Optical fibers with polyimide coatings suppress fiber autofluorescence interference. End-angle polishing and anti-reflective coating of the fiber further reduce background noise.
2. Technical Performance Validation in Multiple Application Scenarios
Using the head-mounted microscope, the team successfully conducted brain function and brain disease imaging experiments in freely moving mice, demonstrating the technology’s potential in neurovascular coupling research.
1) Neurovascular Response to Global Hypoxic Challenge
By reducing the oxygen concentration inhaled by mice to simulate a hypoxic environment, the study found heterogeneous neuronal responses: calcium signals in some neuronal somata significantly increased (△F/F +97%), while others were suppressed (△F/F −49%). Synchronized vascular imaging showed a 36% decrease in overall vascular sO₂ and dilation in most blood vessels (up to 37% increase in diameter). Correlation analysis between neuronal calcium dynamics and sO₂ changes revealed distinct oxygen-dependent characteristics among neuronal populations, enabling classification of different neuronal subtypes.
2) Neurovascular Regulation under Local Somatosensory Stimulation
Rapid electrical stimulation of the limbs in freely moving mice activated corresponding somatosensory cortical neurons, with calcium signal peaks reaching 202% of baseline. Simultaneously, cerebral arteriolar oxygen levels increased by 22% (compared to only 6.7% in venules). High-resolution photoacoustic imaging showed significant arteriolar dilation during electrical stimulation, indicating active regulation by arterioles to maintain regional oxygen reserves, while venular dilation was minimal, primarily reflecting passive responses to upstream blood flow changes. Neuro-vascular adjacency matrix analysis revealed stronger correlations between arteriolar dynamics and neuronal activity.
3) Neurovascular Coupling Characteristics during Epileptic Seizures
In acute epilepsy experiments using pentylenetetrazole (PTZ)-induced mouse models, the team observed oxygen consumption (sO₂ −9.88%) and abnormal vascular dilation (diameter +36%) caused by low-intensity high-frequency neural discharges preceding epileptic bursts. This oxygen consumption and vascular dilation prior to seizure onset may provide a potential temporal window for epilepsy intervention.
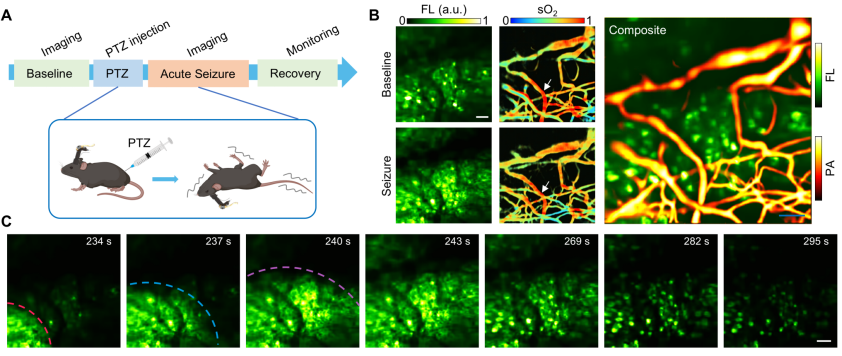
(A) Induction of mouse epilepsy model; (B) Neuronal calcium activity signals and blood oxygen saturation during normal mouse activity and epileptic seizures (left), and representative dual-modal fusion images (right); (C) Recording of epileptic wave propagation process. Scale bar: 50 μm.
3. Future Outlook
This study represents the first synchronized high spatiotemporal resolution imaging of neuronal activity and hemodynamics in the brains of freely moving mice, offering new perspectives for deciphering neurovascular coupling mechanisms and developing next-generation brain-computer interface (BCI) technologies.
Future research will advance on two fronts: imaging technology and BCI applications. On the imaging technology front, efforts will focus on optimizing the head-mounted microscope’s performance—further expanding the imaging field of view, enhancing imaging depth of field and speed, and exploring integration with additional modalities such as two-photon fluorescence microscopy to meet broader research needs. For BCI applications, the team will investigate using head-mounted imaging technology for non-invasive brain function mapping in primates, leveraging neurovascular coupling to precisely decode brain activity. This will provide a scientific basis for developing novel therapeutic strategies and intervention measures for brain diseases such as Alzheimer’s and stroke.