Recently, the team led by Researcher Song Liang and Associate Researcher Liu Chengbo from the Biomedical Optics and Molecular Imaging Laboratory, Institute of Medical and Biological Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, in collaboration with the team led by Professor Jun Zou from Texas A&M University in the United States, has developed a cross-scale photoacoustic microscopy imaging technology based on free-space light transmission and MEMS high-speed scanning imaging. This technology has achieved non-invasive high-speed cross-scale imaging of living small animals from the microscopic to the macroscopic level within the same time scale. The research paper titled "Multiscale high-speed photoacoustic microscopy based on free-space light transmission and a MEMS scanning mirror" was published in *Optics Letters*, a well-known and classic journal in the field of optics sponsored by the Optical Society of America.
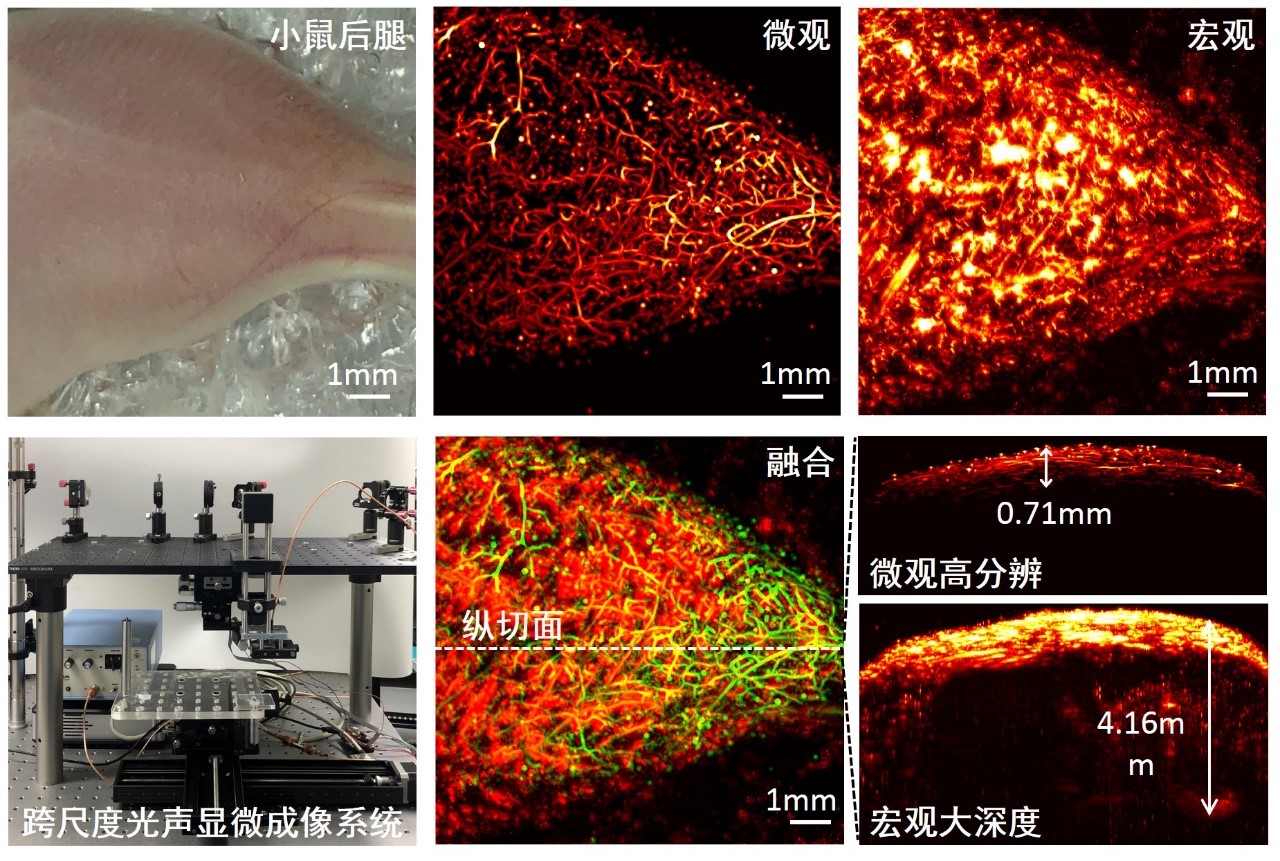
Figure 1. Cross-scale photoacoustic microscopy imaging results of the blood vessels in the hind legs of a mouse
In this study, Chen Zhang, Huangxuan Zhao, and Song Xu (co-first authors of the paper) used the method of transmitting light in free space to achieve two photoacoustic microscopy imaging modalities, optical resolution (OR) and acoustic resolution (AR), in the same system. The developed cross-scale photoacoustic imaging instrument can achieve imaging with different resolutions across multiple tissue depths. At the same time, based on the water-immersed microelectromechanical system (MEMS) developed by the collaborating institution, Texas A&M University, high signal-to-noise ratio and fast imaging were achieved. In the photoacoustic imaging of blood vessels in living animals, the lateral resolution of the system can be flexibly switched between 4.9 µm and 114.5 µm, with imaging depths reaching 0.7 mm and 4.1 mm respectively. The imaging speed and field of view reach 1×1 mm² per second, and three-dimensional depth-resolved information is included. The successful development of this system will play a positive role in tumor mechanism research and brain science research. On this basis, the research team is developing the second-generation system, which can simultaneously obtain structural, functional, and molecular information of organisms. It has the potential to accurately define tumor boundaries, monitor brain blood supply and nerve activities in real time, and study the mechanism of cerebrovascular-nerve coupling. This research is supported by projects such as the National Natural Science Foundation for Excellent Young Scientists, major instruments of the National Natural Science Foundation, major research programs of the National Natural Science Foundation, and instruments of the Chinese Academy of Sciences. Paper link: https://www.osapublishing.org/ol/abstract.cfm?uri=ol-45-15-4312